Development of a single-step, protein A chromatography process for bispecific antibodies in early screening
A single-step downstream process using protein A chromatography to purify a bispecific antibody (BsAb) in early screening has been successfully developed to replace an existing-three step process. The goal was to separate BsAb monomer from fragments such as the individual heavy and light chain pairs. MabSelect PrismA protein A resin was selected for the capture step. By applying a pH gradient elution from pH 5 to 3.5, most of the fragments could be removed as early as the capture step and without the need for additional purification steps. Target BsAb was efficiently purified with high recovery and purity using the single-step process described.
Introduction
BsAb have gained increasing interest over recent years with their wide range of applications such as potential candidates for cancer immunotherapy or as drug delivery vectors (1, 2). There are many types of BsAbs, but they all share two main characteristics:
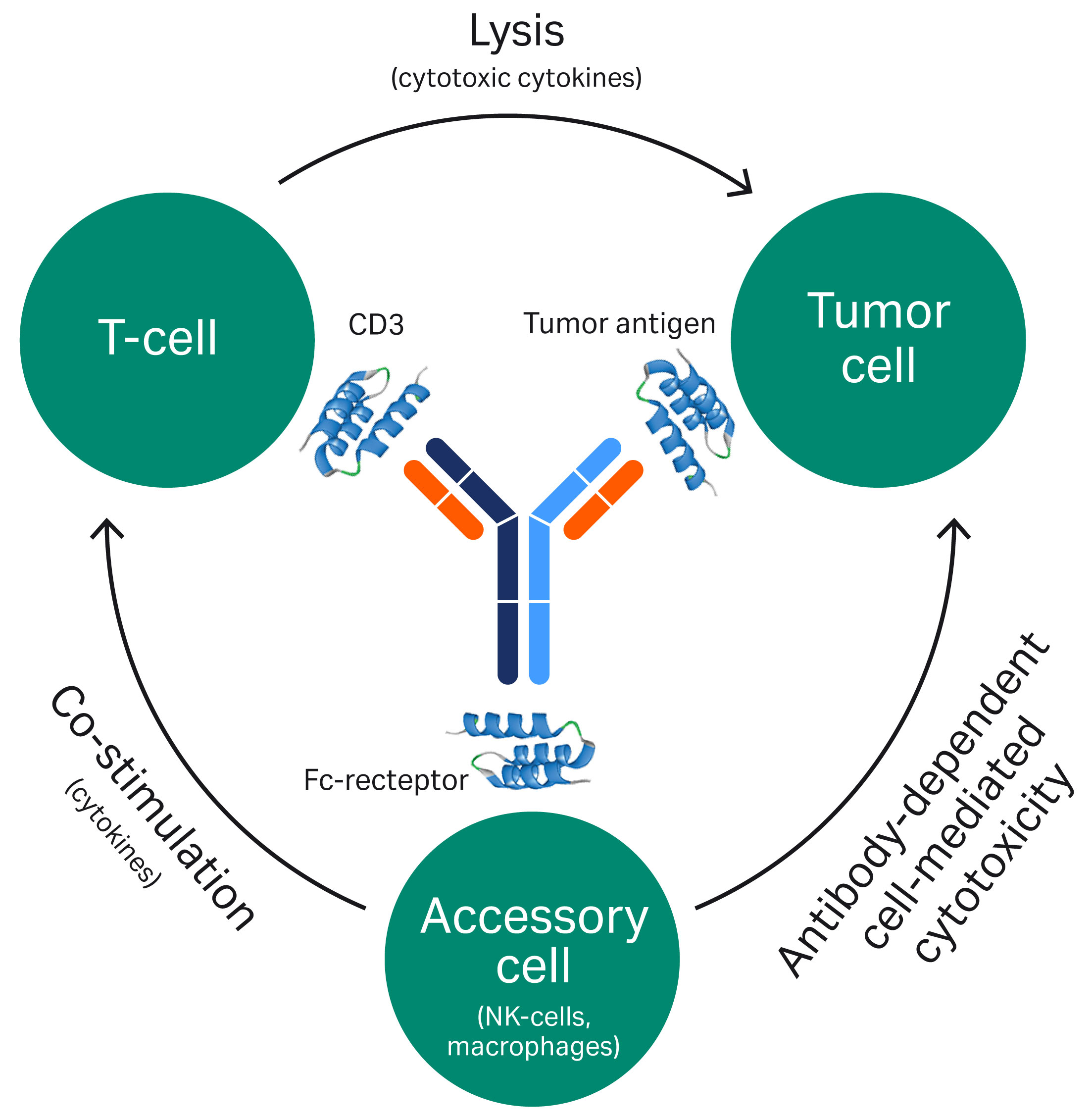
(i) BsAbs are derived from monoclonal antibodies (mAbs)(ii) BsAbs have at least two different sites that specifically target different antigensThe dual specificity means that BsAbs can, for example, bind to target cells using one antigen-binding site and recruit other cells or molecules with the second antigen-binding site (Fig 1).
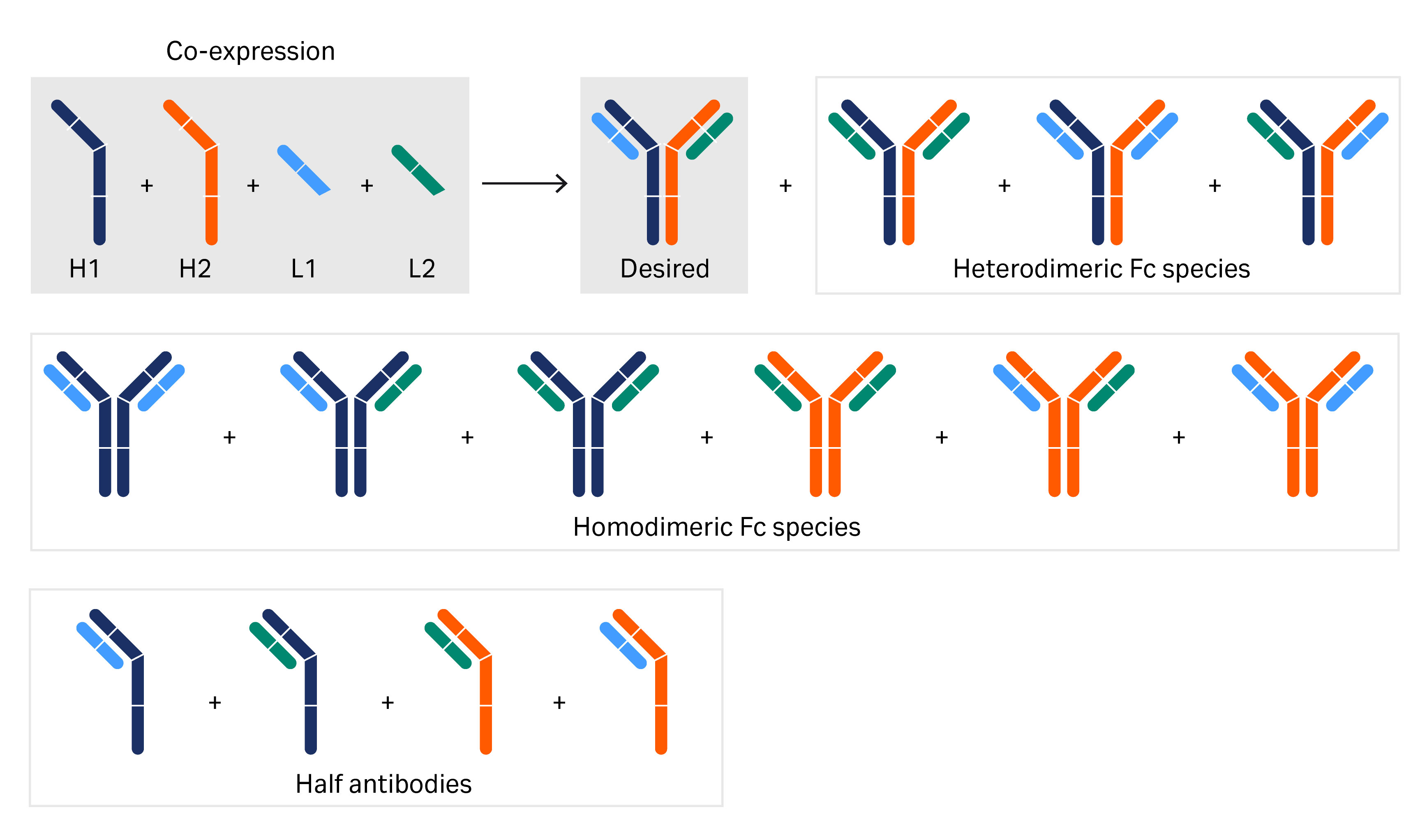
To find relevant lead biotherapeutic BsAb, screening of large numbers of BsAbs is required to find relevant lead candidates (3). A multistep purification process involving an antibody purification resin capture step as well as one or two additional purification steps is typically required to remove undesirable impurities closely related to the BsAb monomer, which originate from the target BsAb (Fig 2). The BsAb used in this study is a three-chain BsAb heterodimer with two different heavy chains (HC) and the same light chain (LC). The different chains are expressed from different vectors and combined to the final BsAb molecule in the cell cytosol. Another challenge is to handle a high number of BsAb candidates produced for further clinical investigations.
Aiming for more efficient BsAb purification with fewer steps
The aim of this work was to provide a rapid process for early screening purification of BsAb candidates from its individual or partly assembled BsAb fragments.
The study performed is a collaboration with Celgene with a view to replacing their three-step affinity chromatography purification protocol with a more rapid method. Celgene’s present method is a several week process for each candidate consisting of a three-step purification: a protein A capture step with MabSelect SuRe, a multimodal chromatography intermediate step with Capto adhere, and a hydrophobic interaction chromatography polishing step with Capto Phenyl ImpRes—all resins from Cytiva.
An alternative one-step approach was devised to shorten time to results while still providing the purity (> 90%) and yield (> 85%) required for manufacturing of BsAb constructs.
Fig 1. An example of a mode-of-action for a bispecific mAb.
Fig 2. The building blocks and the numerous different genetically engineered variants of bispecific antibodies investigated in this article. Various combinations of the heavy- and light-chain regions as well as half antibodies are shown.
Materials and methods
Sample
BsAb-containing cell culture feed was provided by Celgene, Inc. Information about the expression system is proprietary to Celgene, Inc.
Resin screening for the capture step
Three resins from Cytiva were screened for the capture step—MabSelect PrismA, MabSelect SuRe LX, and MabSelect SuRe ppc. Table 1 lists the products and their properties in terms of relative dynamic binding capacity at specified residence times. Conditions for the screening study are listed in Table 2.
Table 1. Resins included in screening for the protein A capture step
| Resin |
Properties |
|
Protein A resin providing high dynamic binding capacity (DBC) at |
|
|
Protein A resin providing high DBC: |
|
|
Protein A resin providing high DBC at short RT: |
Table 2. Running conditions for screening of resin for the protein A capture step
| Sample |
3 mL (0.35 g/L) BsAb-containing cell culture feed |
|
Column volume |
1 mL |
|
Equilibration/binding buffer |
Phosphate buffered saline (PBS) |
|
Wash |
10 CV of 25 mM sodium citrate, pH 6 |
|
Elution |
0% to 100% 25 mM sodium citrate, pH 3 in 20 CV |
|
Flow rate |
0.25 mL/min |
Analysis
BsAb purity was determined by size exclusion chromatography (SEC) on a Superdex 200 Increase 10/300 GL column. Automation of the SEC was achieved using an ÄKTA chromatography system. Peaks were integrated and percentage of whole BsAb, half-antibody fragments, and BsAb aggregates was determined.
Results
Resin screening
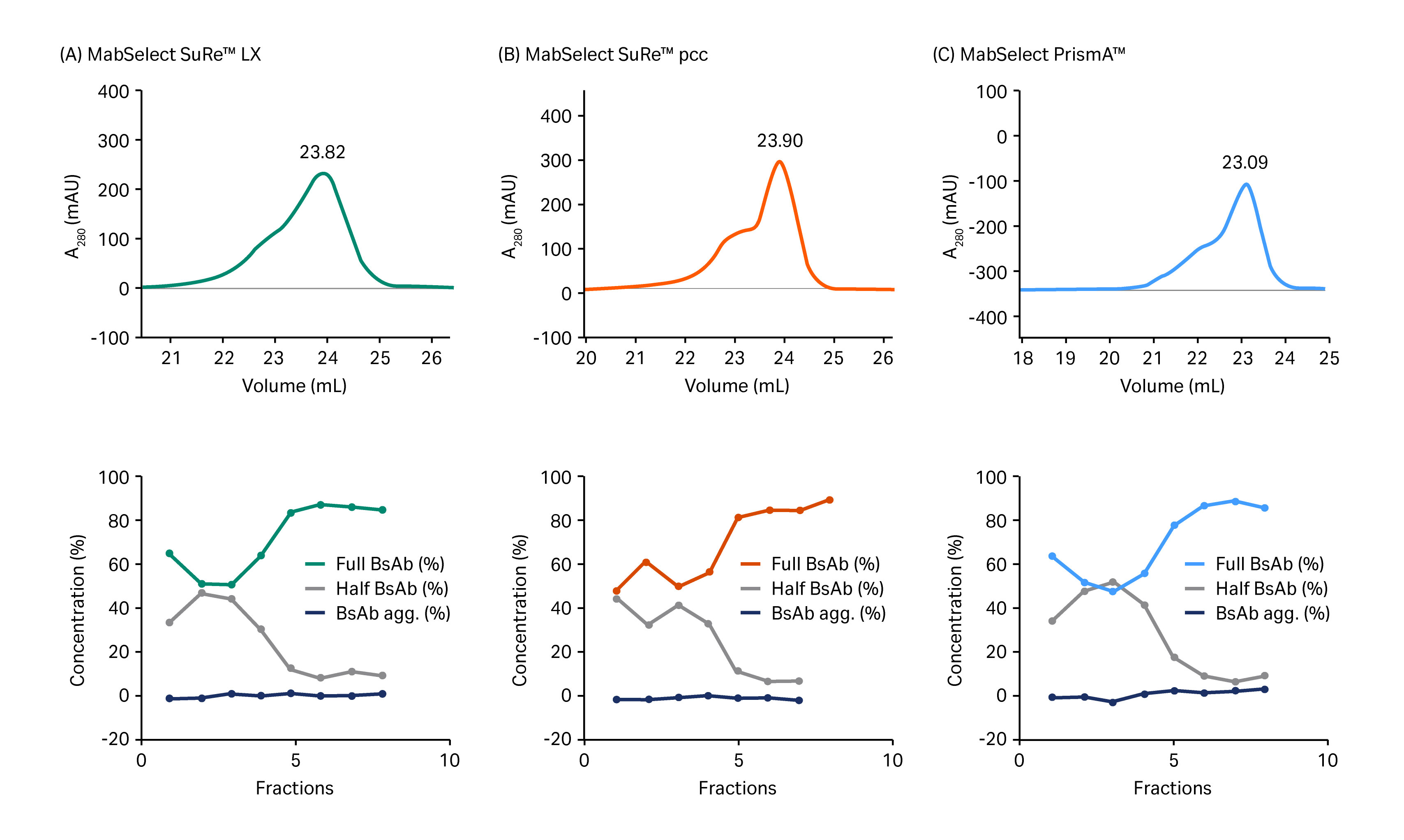
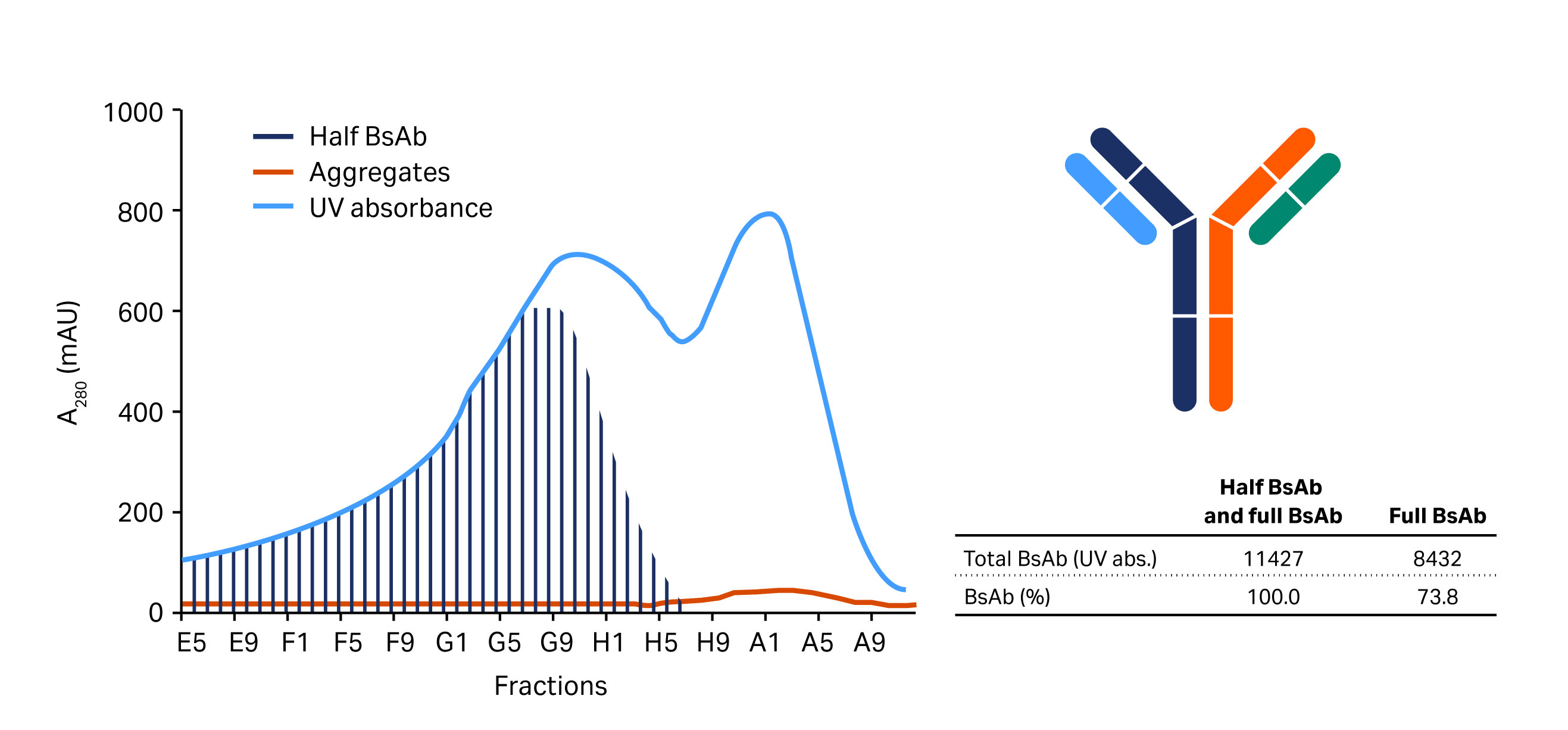
Of the screened protein A resins, MabSelect SuRe pcc has the smallest particle (bead) size (~ 50 µm) followed by MabSelect PrismA (~ 60 µm), and MabSelect SuRe LX with the largest particle size (~ 85 µm). As expected, MabSelect SuRe pcc and MabSelect PrismA offered improved resolution over MabSelect SuRe LX. However, MabSelect PrismA was selected for the capture step due to its higher capacity and alkaline stability than MabSelect SuRe pcc. Figure 3 shows chromatograms from the separations using these resins.
Column: Tricorn 5/50 (1 mL)
Resins: MabSelect SuRe LX, MabSelect SuRe pcc, MabSelect PrismA
Sample: 3 mL (0.35 g/L) of BsAb-containing cell culture feed
Equilibration: PBS
Wash: 25 mM sodium citrate, pH 6
Elution: 0% to 100% of 25 mM sodium citrate, pH 3 in 20 CV
System: ÄKTA avant 25
Fig 3. Upper images show chromatograms from separation of whole BsAb from half-antibody fragments and BsAb aggregates on (A) MabSelect SuRe LX, (B) MabSelect SuRe pcc, and (C) MabSelect PrismA. Lower images show results from analysis by size exclusion chromatography for fractions equivalent to the volume range from 21.5 mL to 25.0 mL in the chromatograms shown in (A).
Process verification
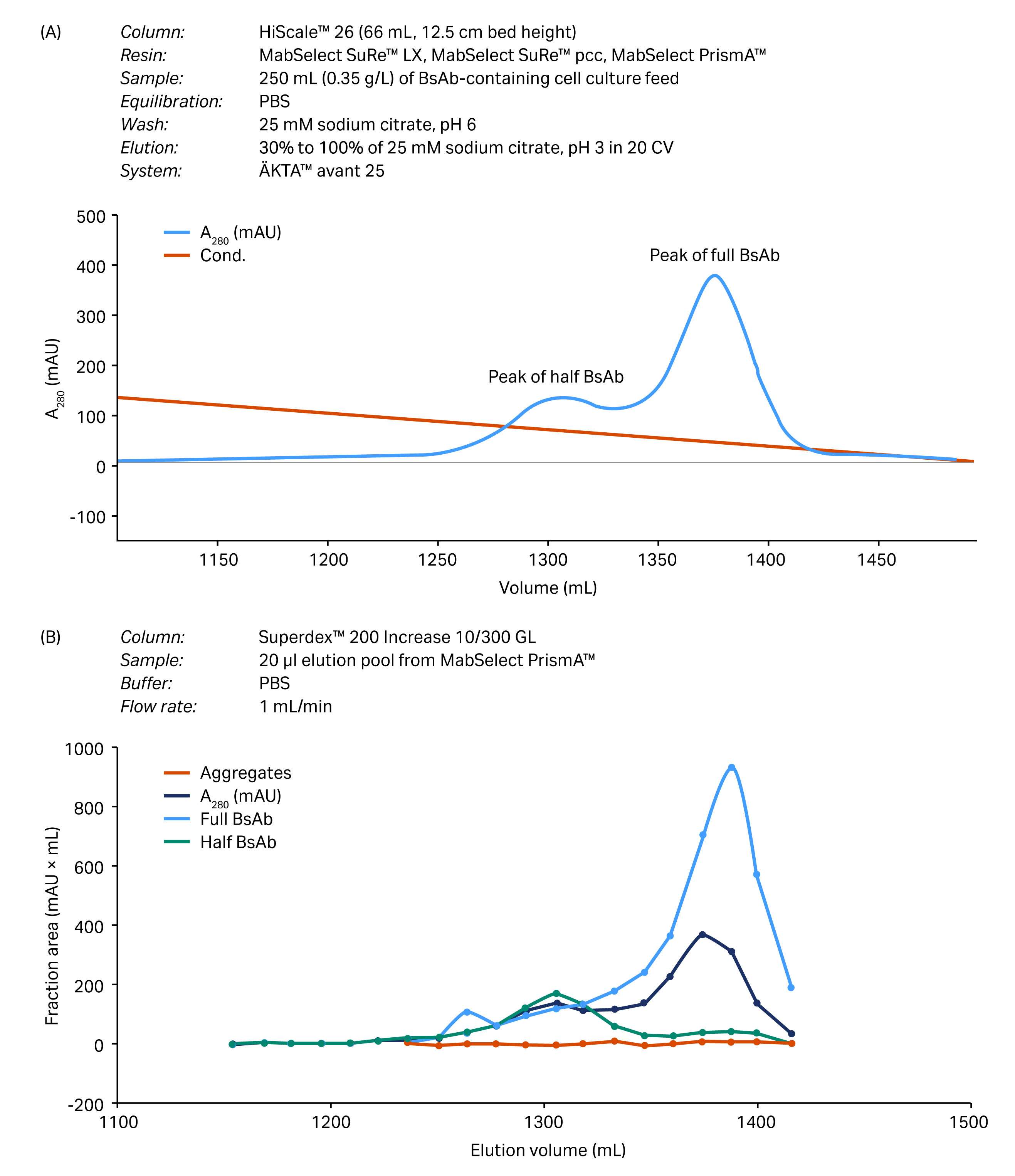
Process verification was evaluated in a larger scale column designed for process development (Fig 4). BsAb purity was approximately 95% at a recovery of 80% as determined by calculation of the area under the curves.
(A)
Column: HiScale 26 (66 mL, 12.5 cm bed height)
Resins: MabSelect SuRe LX, MabSelect SuRe pcc, MabSelect PrismA
Sample: 250 mL (0.35 g/L) of BsAb-containing cell culture feed
Equilibration: PBS
Wash: 25 mM sodium citrate, pH 6
Elution: 30% to 100% of 25 mM sodium citrate, pH 3 in 20 CV
System: ÄKTA avant 25
(B)
Column: Superdex 200 Increase 10/300 GL
Sample: 20 µL elution pool from MabSelect PrismA
Buffer: PBS
Flow rate: 1 mL/min
Fig 4. Process verification of the capture step after scale-up to a HiScale column. (A) Chromatogram from the separation of full BsAb, half BsAb, and BsAb aggregates separated on MabSelect PrismA resin. (B) Results from size exclusion chromatography (gel filtration chromatography) analysis using an analytical SEC column.
The result from the capture step was further elucidated using four more constructs under the same conditions. A chromatogram for one of these constructs is seen in Figure 5.
Fig 5. Verification of the new capture step with the bispecific construct/candidates shown on the right. Elution of this construct is seen in the second peak. The first peak is a half mAb.
Method optimization was performed on a PreDictor 96-well microplate prefilled with MabSelect PrismA resin. Assist software was used for method optimization. Buffer components, pH, additive, and the steepness of gradient were investigated using Assist software. The buffer conditions and resulting yield and recovery of BsAb monomers are shown in Table 3. The flowthrough fractions from the PreDictor 96-well plates were analyzed for monomer concentration, yield, and purity and compared to the control conditions, that is, 25 mM citrate buffer, pH 3 (Table 3).
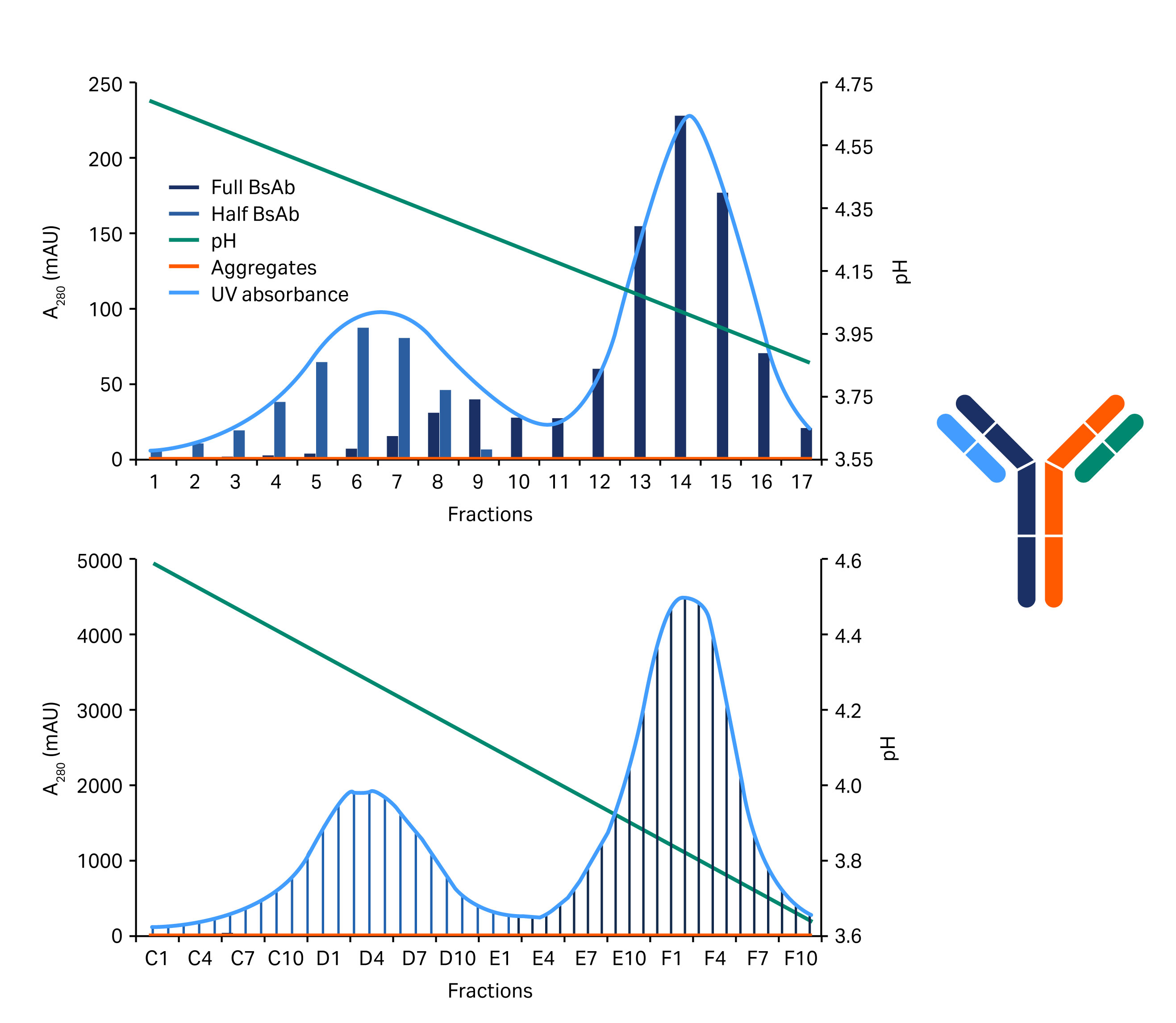
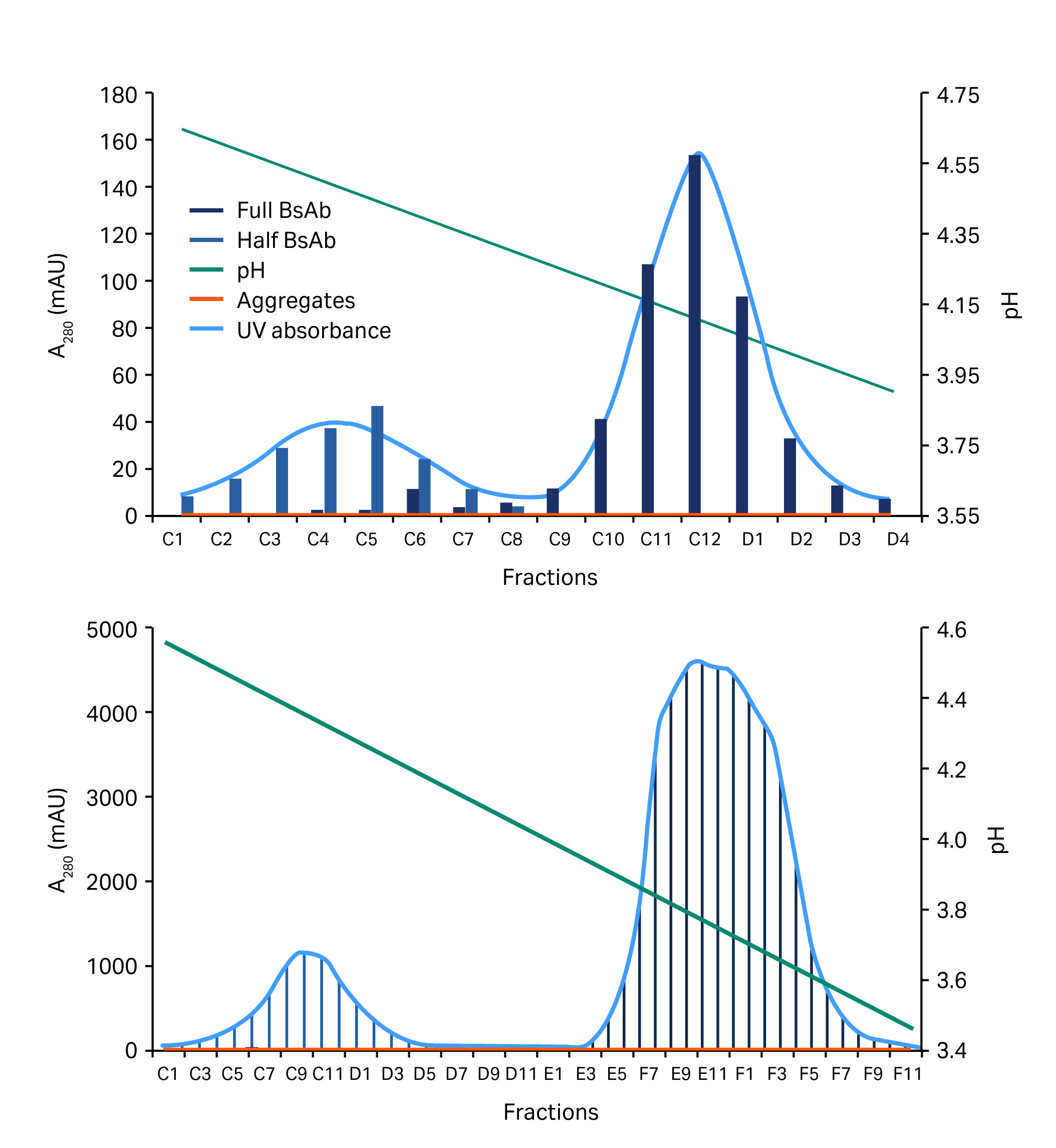
The 0.1 M acetate buffer, pH 3 gave a slightly better result and this was verified in a column run. In this run, a shallower gradient was employed. The total gradient was over 40 CV. This new protocol was applied to a successful purification of two different four-chain constructs at low and high load (Fig 6 and Fig 7).
Table 3. Optimization of buffer conditions, recovery, and yield of BsAb monomer on MabSelect PrismA
| Buffer | BsAb monomer (%) | Monomer (percentage of control, %) | Yield (µg) | Yield (percentage of control) | Total monomer recovery, µg (monomer × yield) | Total monomer recovery (percentage of control) |
|---|---|---|---|---|---|---|
| 25 mM citrate, pH 3.0 | 89.9 | 100 | 55.4 | 100 | 49.8 | 100 |
| 100 mM acetate, pH 3.0 | 96.0 | 107 | 59.0 | 107 | 56.6 | 114 |
| 100 mM acetate, pH 3.5 | 95.0 | 106 | 57.0 | 103 | 54.1 | 109 |
| 100 mM citrate, pH 3.25 | 95.3 | 106 | 58.0 | 105 | 55.2 | 111 |
| 100 mM citrate, 250 mM NaCl, pH 3.25 | 95.0 | 106 | 57.0 | 103 | 54.1 | 109 |
| 100 mM glycine, pH 3.0 | 94.0 | 105 | 56.0 | 101 | 52.8 | 106 |
Fig 6. Chromatograms showing the separation of the half BsAb and the full length BsAb (blue/gray area) variants of the construct shown on the right. The above chromatogram shows low sample load and the below chromatogram shows high load.
Fig 7. Chromatograms showing the separation of a half BsAb and the full length BsAb (blue/gray area) at low (above) and high (below) sample loads.
Conclusions
MabSelect PrismA provided good separation of BsAb from unassembled fragments in the initial capture step. Rapid screening of different protein A resins and elution conditions allowed development of a one-step purification protocol. The new one-step approach resulted in a time-reduction of 4 to 6 wk for each candidate compared to the original three-step process. The suitability of the process was verified with two different constructs and both purity and yield fulfilled the set requirements of > 90% purity and > 85% yield.
Learn more about MabSelect protein A resins.
References
- Fan, G. et al. Bispecific antibodies and their applications. J. Hematol. Oncol. 8, 130 (2015). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4687327/
- Muller, D. and Kontermann, R. E. Bispecific antibodies for cancer immunotherapy: Current perspectives. BioDrugs 24(2), 89–98 (2010). https://www.ncbi.nlm.nih.gov/pubmed/20199124?dopt=Abstract
- Zwolak, A. et al. Rapid Purification of human bispecific antibodies via selective modulation of protein A binding. Sci. Rep. 7, article no. 15521 (2017). https://www.nature.com/articles/s41598-017-15748-0
Acknowledgement
We thank Celgene, Inc. for providing us with the target molecule and for sharing of data.