Lentivirus (LV) is an enveloped RNA virus that is characterized by its ability to incorporate viral RNA into host cell DNA. For this reason, LV vectors (LVV) are used in the cell and gene therapy field for delivery of nucleic acids into target cells both ex vivo and in vivo. Vector production is one of the major contributors to overall manufacturing costs for cell and gene therapies. Therefore, improvements in vector production, such as increased vector yield per batch, are of high importance as it can reduce manufacturing costs and improve the availability of therapies.
We have developed a scalable and robust upstream process for lentiviral vector (LVV) production of HEK293T cells using ReadyToProcess WAVE™ 25 rocker and Xcellerex™ XDR 10 single-use stirred-tank bioreactor. We describe how to expand HEK293T suspension cells from cryovials to small cell culture in shake flasks and up to a 10 L bioreactor culture. The process was closed and based on serum-free culture in HyClone™ peak expression medium. LVV was produced at high titers upon transient plasmid transfection in the bioreactor.
Introduction
In recent years, there has been a shift from adherent culturing towards serum-free suspension conditions to improve cell culture processes. Here, we utilized a design of experiment (DoE) methodology to optimize LVV production of a HEK293T suspension cell system and we performed the experiment in separate stages:
- First, we used DoE to evaluate the transfection protocol in shake flask, this allowed us to evaluate the impact of multiple factors key to scaling LVV production up, such as plasmid concentration, cell densities, and harvest time.
- Next, we performed LVV production in ReadyToProcess WAVE™ 25 rocker or Xcellerex XDR 10 single-use stirred-tank bioreactor using the protocol established in the first part.
An efficient, robust scalable cell culture process for LVV production was verified by triplicate runs using ReadyToProcess WAVE™ 25 rocker and Xcellerex™ XDR 10 bioreactor system for LVV production.
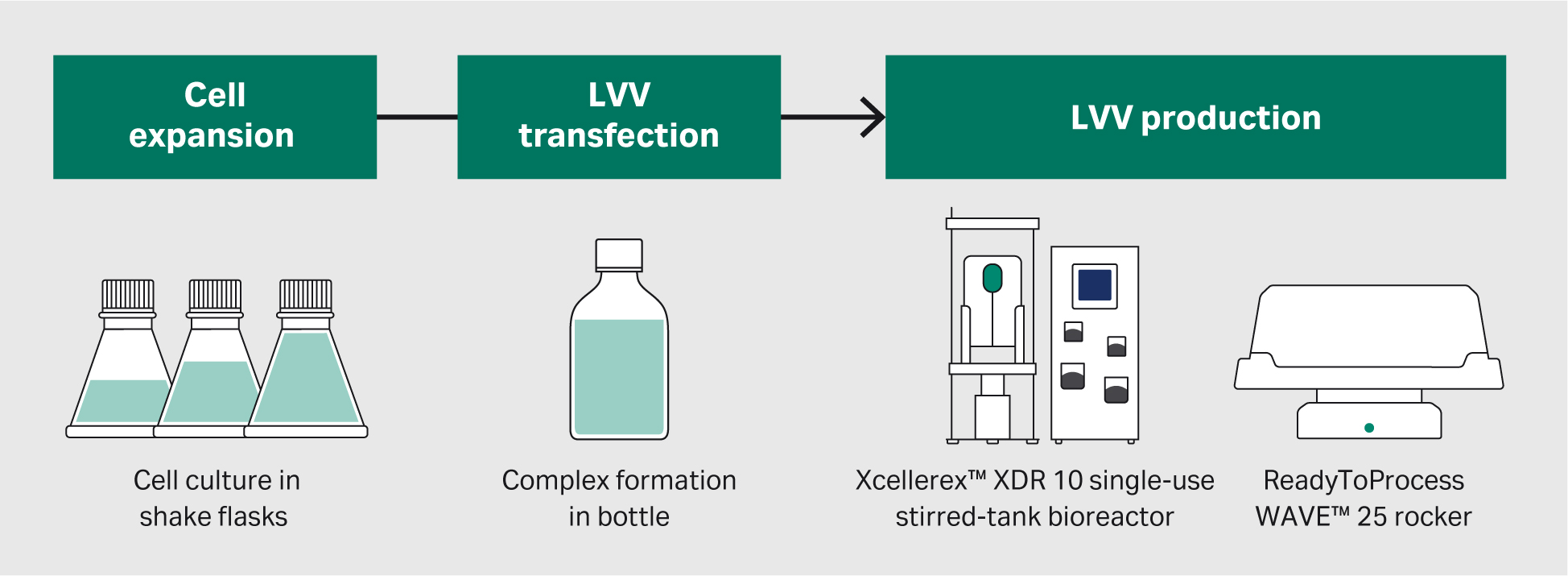
We performed the production process in different steps (Fig 1):
Fig 1. An overview of the LVV production process. We will discuss the cell culture and transfection process development in this article.
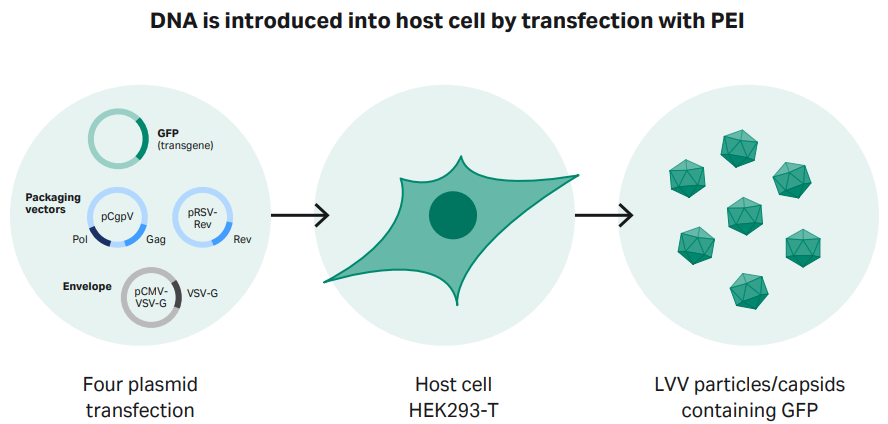
To produce LVV, we used a four plasmid transfection system (Cell Biolabs) consisting of two packaging vectors, one envelope vector, and a transfer vector carrying the green fluorescent protein (GFP) reporter gene, along with PEI MAX (Polysciences) transfection reagent. PEI creates positively charged complexes with plasmid DNA that enters the cell via endocytosis and delivers the DNA into the nucleus, resulting in gene expression of the exogenous DNA (Fig 2).
Fig 2. Transient transfection of HEK293T cells using a four-plasmid transfection approach. Polyethyleneimine (PEI) forms a complex with DNA, which is then introduced into the host cells.
Detailed Materials and methods are found at the end of this article.
Results
Adaptation of HEK293T cells to a serum-free process for LVV production
First, we adapted adherent HEK293T cells to HyClone™ peak expression cell culture transfection medium. Adaptation was performed by transferring cells grown in classical HyClone™ DMEM medium with high glucose supplemented and 10% HyClone™ Fetal Bovine Serum (FBS) directly into the new transfection medium. HyClone™ peak expression medium is ready-to-use and contains stable L-glutamine and poloxamer 188. The cell growth and morphology of the HEK293T suspension cells were evaluated visually over 10 passages. Finally, we created research and working cell banks (RCB and WCB, respectively) from cryopreserved cells grown in the HyClone™ peak expression medium.
Plasmid constructs for LVV process development
Amplification of plasmids for LVV production was performed by transforming bacteria with plasmids. The plasmid DNA was subsequently replicated upon culturing the transformed bacteria. By using a plasmid purification kit, plasmid DNA was retrieved from the harvested material.
Optimized LVV production protocol using HEK293T suspension cells
The DoE study was designed as a full factorial design, including five parameters (VCD, incubation time, enhancer concentration, enhancer time, and DNA concentration), to evaluate the optimal conditions for transfection. During our process development, the goal was to achieve the following criteria: 1010 virus particles (VP/mL) and 107 transducing units (TU/mL).
It was found that all conditions resulted in a viral titer that exceeded the acceptance criteria set for the project.
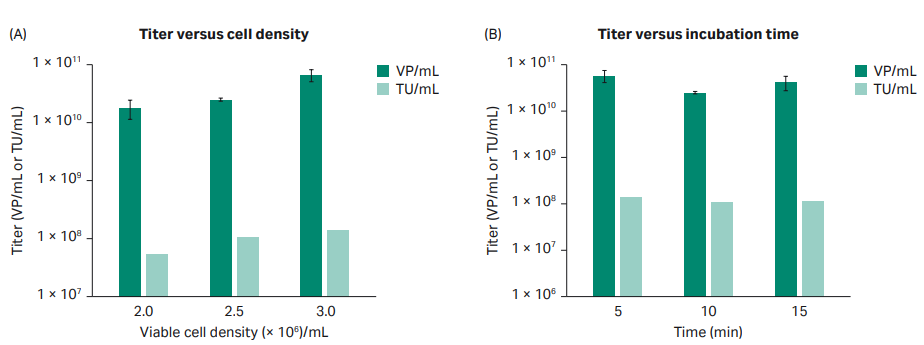
Instead of performing the additional DoE needed to resolve the curvature for the VCD, a simple additional experiment was carried out where only three different VCD and incubation times were tested with all other parameters set according to results from the DoE. The results from the experiment concluded that the cell density should be 3 × 106 viable cells/mL and that the incubation time should be 5 min to maximize titer (Fig 3).
Fig 3. Evaluation of VCD and incubation time. All flasks evaluated for VCD had a DNA-PEI complex incubation time of 10 min. All flasks evaluated for incubation time had a VCD of 2 × 106 cells/mL.
Optimized transfection protocol
After the initial DoE study had been performed, a protocol was designed for production of LVV based on the results (Table 1).
Table 1. Final parameters used for the transfection protocol
| Transfection parameters |
|
|---|---|
| Cell line | HEK293T, suspension adapted |
| Cell culture medium | HyClone™ peak expression |
| Transfection reagent | PEI MAX 40k |
| Plasmid Ratio | 1:1:1:3 (1 pRSV-Rev: 1 pCMV-VSV-G: 1 pCgpV: 3 pGFP) |
| VCD at transfection (viable cells/mL) | 3 × 106 |
| DNA (µg/mL) | 1 |
| DNA:PEI ratio (µg:µL) | 1:2 |
| Transfection solution incubation time (min) | 5 |
| Sodium butyrate addition (h post transfection) | 5 |
| Sodium butyrate concentration (mM) | 10 |
| Time of harvest (h post transfection) | 72 |
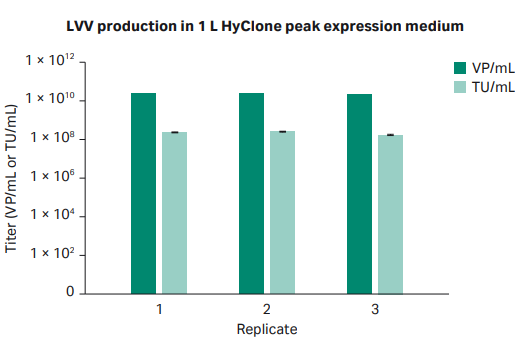
To confirm the transfection protocol for LVV production, 1 L of LVV was produced in triplicate in shake flasks using HyClone™ peak expression medium. The virus titer in the harvest material was above the acceptance criteria for all replicates, and in addition, the variance between replicates was small (Fig 4).
Fig 4. Infectious and viral particle titer from production of 1 L LVV in shake flasks using HyClone™ peak expression medium.
Production in Xcellerex™ XDR 10 single-use stirred-tank bioreactor
First, HEK293T cells were expanded in shake flasks to produce sufficient cells for an inoculation at a cell concentration of 0.4 × 106 cells/mL in a 5 L starting volume for the Xcellerex™ XDR 10 single-use stirred-tank bioreactor. Xcellerex™ XDR 10 bioreactor was set up for inoculation and growth of HEK293T suspension cells to a density of approximately 3 × 106 cells/mL. Cells were diluted by addition of HyClone™ peak expression medium after 72 h to reach approximately 9.2 L.
Transfection was performed 96 h post inoculation and according to our optimized protocol. The transfection complex solution was added to the bioreactor with a final bioreactor volume of 10 L and a cell concentration of 3 × 106 cells/mL. After 5 h post transfection, an addition of Sodium Butyrate was done at a final concentration of 10 mM. Harvest was done 72 h post transfection.
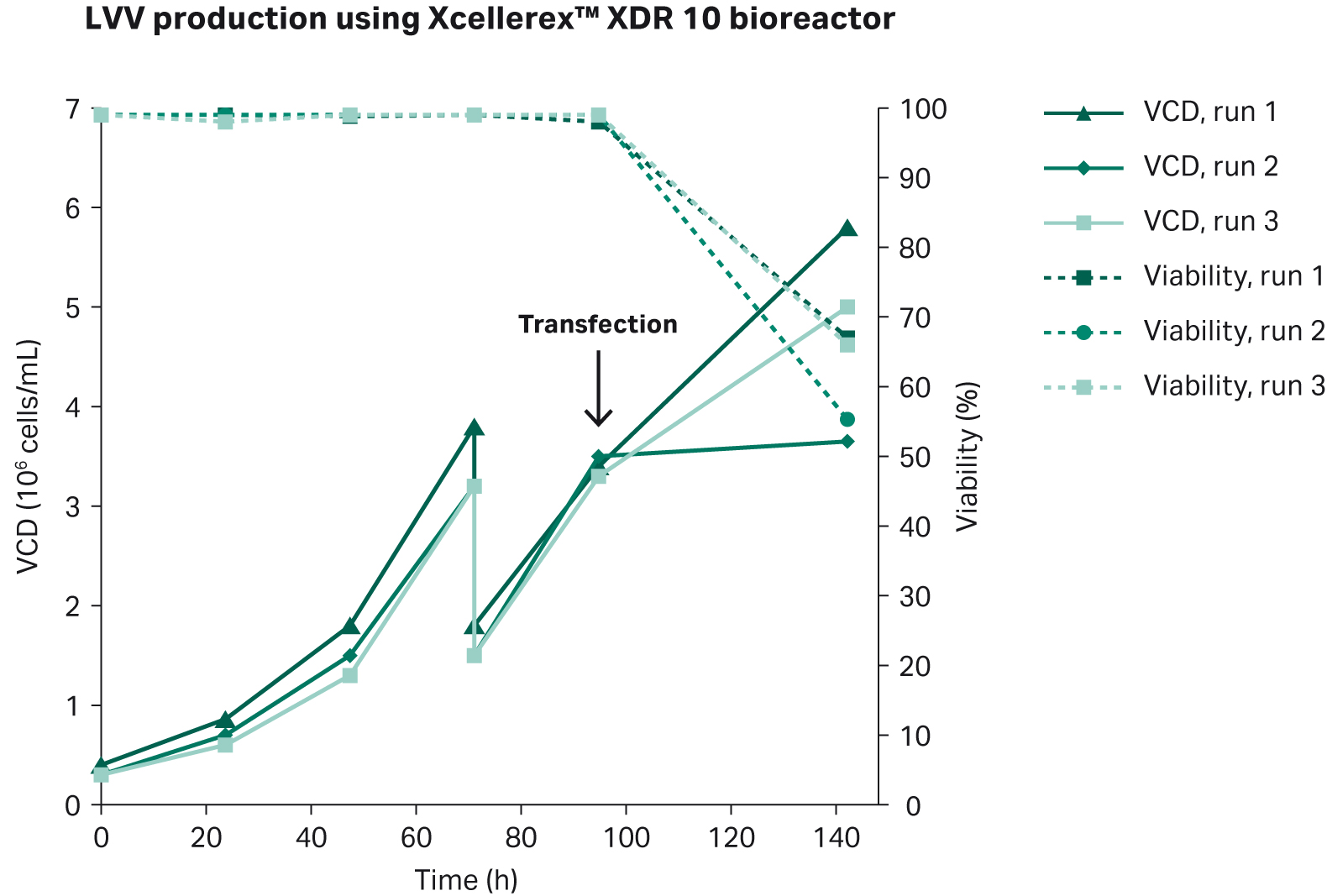
The results from the three production batches in Xcellerex™ XDR 10 bioreactor are shown in Figure 5. All three production runs met the acceptance criteria for viral titer, although difference between runs could be observed. Notably, the largest difference between runs can be observed at the time of harvest, and the reason for one run displaying a lower cell density is most likely due to sampling at harvest.
Fig 5. Cell growth and viability in triplicate production runs of LVV in Xcellerex™ XDR 10 bioreactor. Transfection was done 96 h post inoculation, and harvest was done 72 h post transfection.
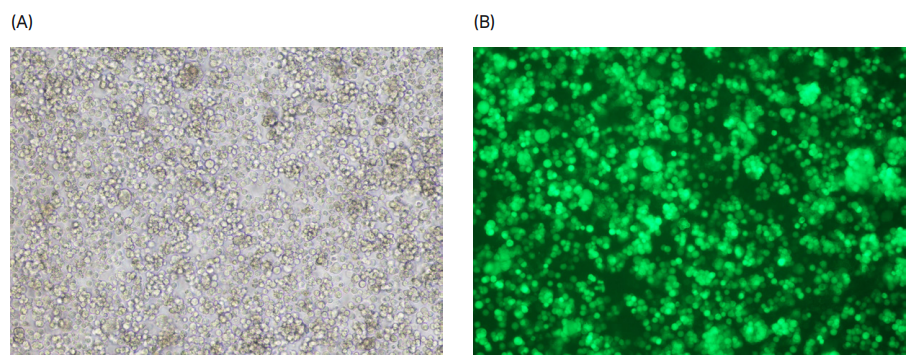
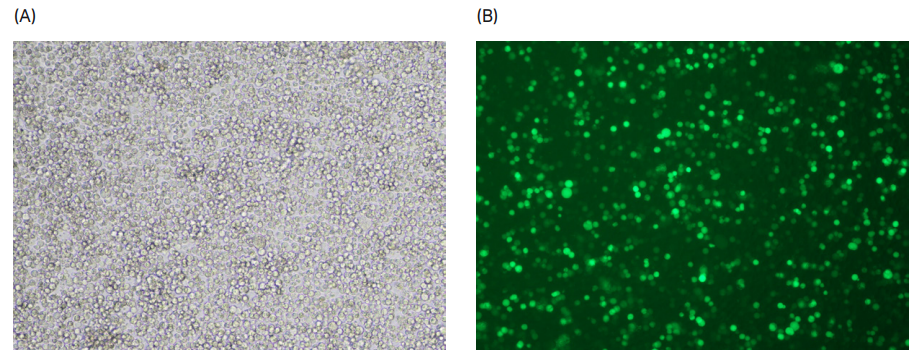
To confirm transfection efficiency in the Xcellerex™ XDR 10 bioreactor, we performed brightfield and fluorescent microscopic evaluation of cells in addition to flow cytometry analysis. Figure 6 shows GFP-positive fluorescing cells in diluted sample of LVV at harvest.
Fig 6. (A) Bright field and (B) fluorescent microscope images of LVV at harvest from Xcellerex™ XDR 10 bioreactor.
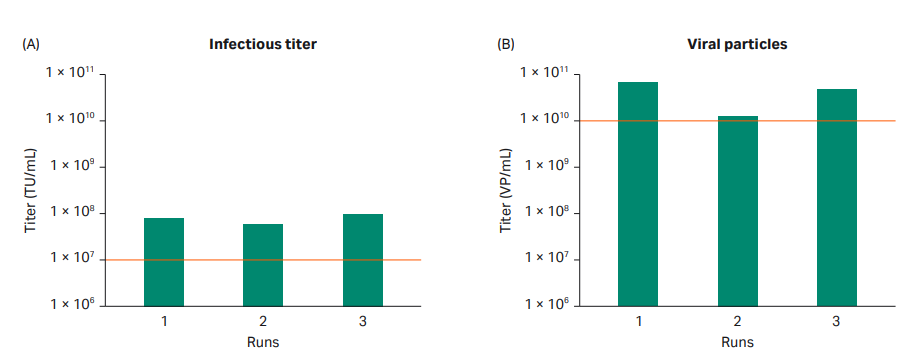
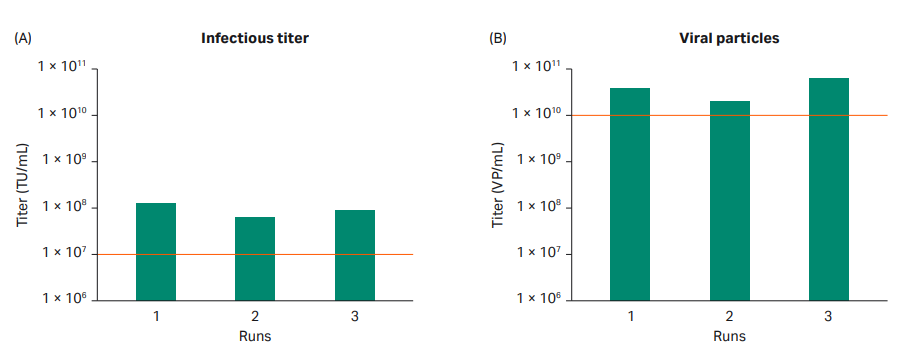
The production batches gave similar viral titers, with approximately 1010 VP/mL and 107 TU/mL in all three production batches (Fig 7). The consistency between all three batches indicated that the process design was robust and suitable for manufacturing purposes.
Fig 7. Viral titer from triplicate production runs of LVV using Xcellerex™ XDR 10 bioreactor. The acceptance criteria for the project are highlighted with dotted red lines. All production runs met the acceptance criteria.
Production in ReadyToProcess WAVE™ 25 single-use bioreactor
Finally, we used ReadyToProcess WAVE™ 25 rocker for the production of 5 L of LVV. HEK293T cells were expanded in shake flasks to produce an inoculation cell concentration of 0.3 × 106 cells/mL in a 2.5 L starting volume. Then, the ReadyToProcess WAVE™ 25 bioreactor was set up for inoculation and growth of the HEK293T suspension cells to a density of approximately 3 × 106 cells/mL. Cells were diluted by addition of HyClone™ peak expression medium after 72 h to reach a volume of approximately 4.8 L.
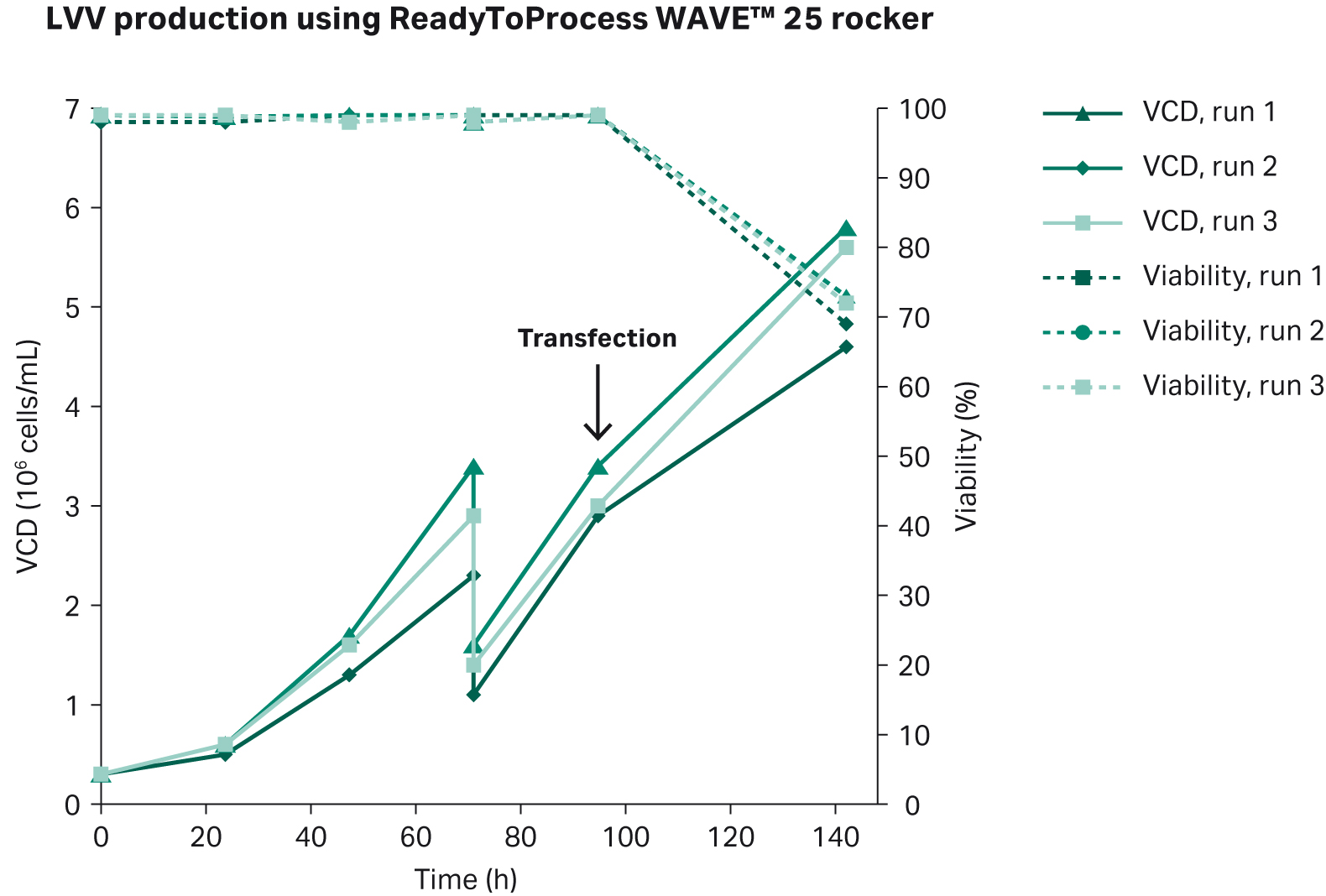
Transfection was performed 96 h post inoculation according to our optimized protocol. The transfection complex solution was added to the bioreactor with a final bioreactor volume of 5 L and a cell concentration of 3 × 106 cells/mL. After 5 h post transfection, an addition of sodium butyrate was done at a final concentration of 10 mM. Harvest was performed 72 h post transfection. The results from the three production batches in ReadyToProcess WAVE™ 25 rocker is shown in Figure 8.
Fig 8. Cell growth and viability of triplicate production runs of LVV using ReadyToProcess WAVE™ 25 rocker bioreactor system. Transfection was done 96 h post inoculation, and harvest was done 72 h post transfection.
To confirm transfection efficiency in the ReadyToProcess WAVE™ 25 rocker, we performed brightfield and fluorescent microscopic evaluation of cells in addition to flow cytometry analysis. Figure 9 shows GFP-positive fluorescing cells in diluted sample of LVV at harvest.
Fig 9. (A) Bright field and (B) fluorescent microscope images of LVV at harvest from the ReadyToProcess WAVE™ 25 rocker.
The production batches gave a similar viral titers, with approximately 1010 VP/mL and 107 transducing units (TU/mL) in all three production batches (Fig 10).
Fig 10. Viral titer results from triplicate production runs of LVV in ReadyToProcess WAVE™ 25 rocker. The acceptance criteria for the project are highlighted with dotted red lines. All production runs met the criteria.
Conclusions and discussion
We have presented a scalable and robust upstream process for LVV production with Hyclone peak expression medium using HEK293T cells in suspension, ReadyToProcess WAVE™ 25 rocker and Xcellerex™ XDR 10 single-use stirred-tank bioreactor. In addition, we have shown:
- Each production run met our acceptance criteria set for the titer in the harvest material (≥ 1010 VP/mL and ≥ 107 TU/mL).
- The protocol also showed scalability from small-scale in shake flasks up to 10 L scale in the Xcellerex™ XDR 10 bioreactor.